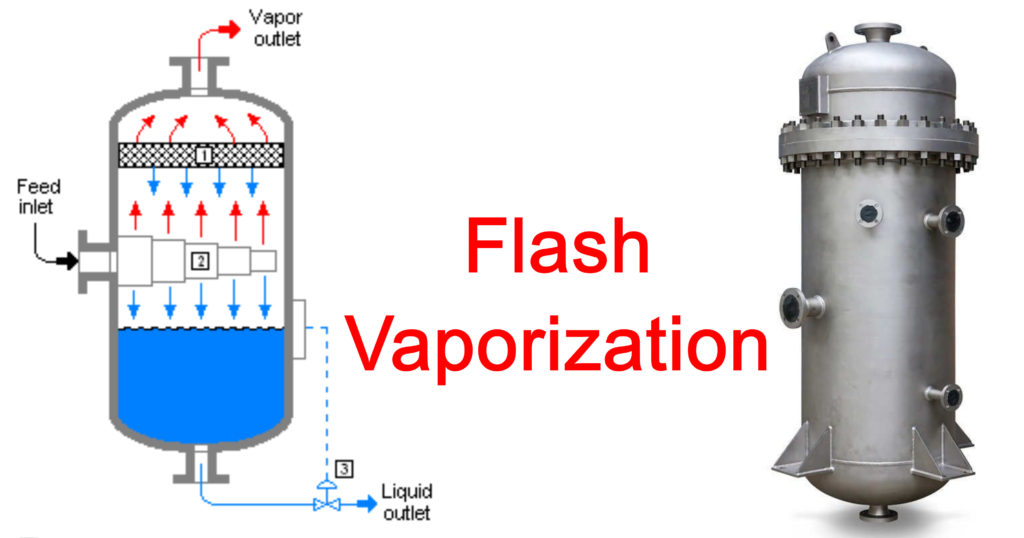
It is a simple unit operation in which a heated liquid mixture is throttled through a valve in order to vaporize the liquid mixture.
The vaporization is done in order to separate the constituents of the liquid mixture. Flash vaporization can be seen as a single stage distillation process.
First, the Feed, which can be a mixture of constituents in liquid phase or in partially vapour-liquid phase, is heated in order to increase its temperature.
In the figure it can be seen that the feed is made to pass through a heat exchanger thus the temperature of the feed can be easily controlled as per requirement and utility.
Then the heated feed is made to pass through a throttling valve. The purpose of the throttling valve is to partially vaporize the feed passing through it.
If the feed is a liquid mixture then vapours are produced and if partially vaporized feed is passed through the valve then more vapours are produced.
If we consider the feed material which is present inside the valve as a system, we see that there are no devices present which lead to work interactions across the boundaries of the system.
In a throttling process it is assumed that the system is Figure: Schematic of a Flash distillation unit adiabatic, meaning that there is no heat interaction across the boundaries of the system.
When we apply energy balance on the system we deduce the following equation,
U + PV = Constant
Here, U is the internal energy, P is the pressure and V is the specific volume.
Also, H = U + PV, H is enthalpy, we find that H = Constant
It means that a throttling process is a constant enthalpy process or isenthalpic process.
If internal energy (U) is assumed to remain constant then we can rewrite the equation as PV = Constant. P and V are the only variables which can change and they will have to change accordingly in order to keep the product of the terms as a constant.
In throttling process the Pressure (P) decreases, it means the specific volume (V) has to increase. Since the liquid is heated to a higher Temperature (T), the pressure decreases and reaches a value which corresponds to the saturation temperature after which vaporization occurs.
The partially vaporized feed is then passed to a flash drum; the pressure of the drum is adjusted accordingly so that some more separation also occurs inside the Flash drum.
During separation some quantity of liquid may get carried away along with vapour, this is called entrainment. In order to separate the entrained droplets there are De-entrainment mesh pads inside the drum.
The vapour is obtained from the top of the drum, it is called top product and liquid is collected from the bottom, it is called bottom product.
It is often assumed that the vapour phase and liquid phase obtained are in equilibrium with each other.
If we assume the feed is a binary mixture made of components A and B then the notations in the figure indicate (Assuming A is more volatile component):
F is the molar flow rate of feed stream.
D is the molar flow rate of top product.
W is the molar flow rate of top product.
ZF is the mole fraction of component A in feed.
XD is the mole fraction of component A in top product.