ETP Effluent Treatment Plant terms blog contains details about Biochemical oxygen demand, biological oxygen demand, chemical oxygen demand, biodegradability, dissolved oxygen, dissolved oxygen sag curve, wastewater treatment, surface water, water quality, BOD, NBOD, EPA, OECD
Blog Content
1. Introduction
2. Theory
2.1. Five-day BOD (BOD5)
2.2. Ultimate BOD (UBOD)
2.3. Carbonaceous Oxygen Demand (CBOD)
2.4. Nitrogenous Oxygen Demand (NBOD)
1.Introduction
As the populations of many cities grew significantly larger during the late 19th Century due to industrial expansion, sewer systems were installed to transport domestic wastewater (from toilets, washing, etc.) and industrial wastewater to rivers or other surface waters for disposal with little or no treatment (see Water Quality, Chemistry of Wastewater, Thermal Pollution of Water).
Primary wastewater treatment, that employed only sedimentation basins, removed large debris and readily settle able solids; however, the majority of the organic material was not removed because it was either dissolved or of low density so that it settled slowly.
Thus, as human populations increased, so did the loading of organics to the nearby surface waters. The increased organic loading stimulated microbial decomposition that utilized dissolved oxygen (DO) in the surface water.
This consumption of DO and attendant DO depletion in many cases led to the development of anaerobic conditions that could not support desired aquatic life, such as fish, and also caused aesthetic water quality problems (see Eutrophication and Algal Blooms).
Advanced (secondary) wastewater treatment was then introduced to biologically remove the organic matter to alleviate this problem. The depletion of dissolved oxygen thus became a primary water quality concern after more important priorities such as disinfection (pathogen destruction) were addressed.
During the period from 1950 to 1970 many industrialized nations passed legislation that aimed to reduce surface water pollution. As a result, wastewater treatment facilities were issued permits which established maximum allowable levels of oxygen demanding wastes (and other contaminants such as suspended solids) in their effluents.
 |
| Treated domestic wastewater being discharged into a stream |
Biochemical oxygen demand (BOD) is also sometimes referred to as biological oxygen demand, but the latter term is considered inappropriate by many scientists and engineers. These terms are widely used to define the microbial use or consumption (i.e. demand) of oxygen during the aerobic oxidation of electron donors such as readily degradable organic carbon (e.g. sugars) and ammonia in waters as shown in the following simplified reactions:
Natural sources of BOD in surface waters include organic material from decaying plants and animal wastes. Human sources of BOD include feces, urine, detergents, fats, oils and grease, etc.
Proteins are produced by plants and utilized by animals. Through the microbial processes of proteolysis, deamination and ammonification proteins are degraded to a hydrocarbon skeleton and ammonia—the two primary chemical forms contributing to BOD as presented in the above equations.
The subsequent biochemical oxidation of these reduced nitrogenous and carbonaceous compounds in water is mediated by a variety of microorganisms (primarily bacteria and protozoa)
Regarding wastewaters, BOD is often used as a measure of the strength of the waste— the greater the BOD, the more “concentrated” the waste. BOD is somewhat unique in that it measures an impact on the environment (mass of dissolved oxygen consumed per volume of water sample— mgO2 L-1), rather than a concentration of any specific compound or family of compounds (e.g., total organic carbon or ammonia).
Measurement of BOD in raw (influent) and treated (effluent) wastewaters is a standard practice to evaluate treatment facility performance. BOD is also one of the primary surface water quality parameters (see Water Quality, Chemistry of Wastewater).
2.Theory
A number of tests have been developed to quantify the BOD, as well as to estimate the rate of oxygen depletion in water or wastewater samples.
This oxidation rate is commonly used in wastewater treatment and surface water quality models.
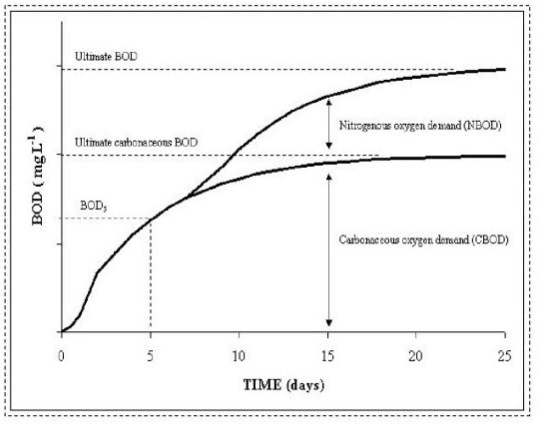 |
| A theoretical representation of the biochemical oxygen demand of a wastewater as a function of time |
Figure diagrams the theoretical aspects of the biochemical oxygen demand of a wastewater sample as a function of time.
Note that the oxygen demand (sometimes referred to as the BOD exerted) increases with time, asymptotically approaching an ultimate value. The inverse of the BOD exerted curve would represent the BOD (degradable organics) remaining in the sample, that exponentially approaches zero.
2.1. Five-day BOD (BOD5)
The BOD5 test is a standardized test that provides information regarding the organic strength of wastewater. The amount of oxygen consumed in a sample within a five-day period is measured under carefully controlled and standardized conditions.
Details of the test are described in Section 3. Generally the five-day period is not long enough for complete oxidation, but it provides sufficient time for microbial acclimation (lag-phase growth as seen during the first day in Figure 2) and for substantial (approximately 40 to 80 percent) oxidation.
The five-day period has been widely retained, having its historical roots in early water quality studies when it was determined that no stream in England had a travel time of greater than five days to the ocean.
The [ BOD5 ], expressed as mgO2 L-1 (or equivalently as parts per million, ppm), is the difference between the initial dissolved oxygen ([DO]) measurement and the corresponding (final) measurement made on the fifth day of incubation.
2.2. Ultimate BOD (UBOD)
The ultimate biochemical oxygen demand ([UBOD]) is a parameter that quantifies the oxygen required for the total biochemical degradation of organic matter by aquatic microorganisms.
[UBOD] and the rate of oxygen consumption are frequently used in mathematical models to predict the impact of an effluent on receiving bodies such as lakes and rivers. The rate of oxygen consumption is therefore often determined along with the [UBOD] value in the analytical test.
A distinction is made between the carbonaceous oxygen demand ([CBOD]) and nitrogenous oxygen demand ([NBOD]) during the measurement as well as in many water quality models. Both CBOD and NBOD contribute to the overall UBOD, but the values and rates of oxidation differ (see Water Quality).
2.3 Carbonaceous Oxygen Demand (CBOD)
where [CBOD] is carbonaceous biochemical oxygen demand remaining, usually
in mgO2 L-1, k is
the first-order reaction rate constant, usually d-1, and [DO] is
dissolved oxygen concentration in mgO2 L-1. This equation
can be integrated resulting in the following:
The value of the reaction rate constant, k, is determined experimentally or from tabulated values. Readily degradable wastes (e.g., domestic wastewater) will have higher (faster) coefficients (0.3 to 0.7 d-1) whereas less readily degradable sources (e.g., river water) will have lower rates (0.1 to 0.2 d-1). An assumption when estimating the ultimate CBOD value is that nitrogenous compounds are inhibited and do not contribute to the overall oxygen consumption.
2.4.Nitrogenous Oxygen Demand (NBOD)
NBOD is the oxygen consumed during the oxidation of nitrogenous compounds (mainly NH3 ) to nitrate with nitrite being an unstable intermediate.
Different from CBOD, only two classes of bacteria are believed to be responsible for the oxidization of reduced nitrogen. These bacteria (nitrifiers) are surface-based (associated with suspended solids), and therefore are usually only present in the water in low concentrations.
The standard analytical test may result in incorrect results because the growth of nitrifiers on the surface of the sample bottle, known as bottle effects, can artificially enhance the nitrification. For this reason, a short-term measurement (1 to 3 days) is suggested to estimate [NBOD].
An accurate method to measure [NBOD], is to track the ammonia (or total Kjeldahl nitrogen, TKN, as a surrogate) concentration over a 1 to 3 day period. [NBOD] (and rate of oxygen consumption) is estimated using the stoichiometric value of 4.57, although a lower value has also been used since some of the nitrogen is consumed for cell maintenance. The rate of oxygen demand in samples can thus be calculated:
where kn is nitrification rate (typically d-1). Integrating and solving the above equation results in
Attempts have been made to measure the [CBOD] and [NBOD] rates simultaneously, but this often results in an incorrect BOD value due to bottle effects (see Section 3.2). The UBOD value should rather be expressed and calculated as the sum of the [NBOD] and [CBOD]:
This equation in many forms is commonly used in dissolved oxygen components of wastewater, lake and river models.









