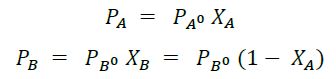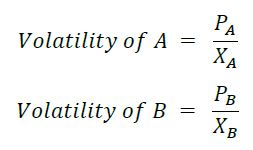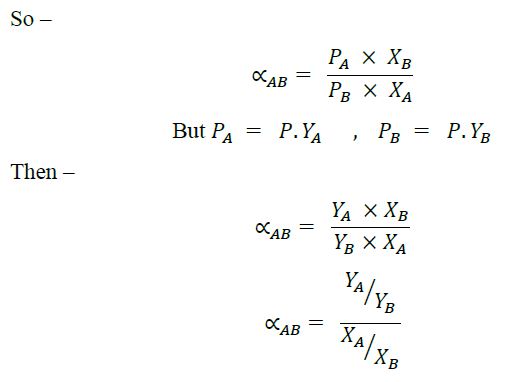Roult's Law:
For an ideal solution equilibrium partial pressure of a constituent at a given temperature is equal to the product of its vapour pressure in pure state and its mole fraction in liquid phase.
Thus for a binary (two components) system if, Pa is the equilibrium partial pressure of A, is vapor pressure of ‘A, Pa0 in pure state and Xa is the mole fraction of A in liquid phase.
PB= equilibrium partial pressure of B.
XB= mole fraction of B in liquid phase
PB0= vapour pressure of B
Dalton's Law:
It state that the total pressure exerted by gas or vapour mixture is equal to the sum of the partial pressure of components percent in it, thus it expresses the additivities nature of partial pressure.
For binary system-
P = Pa + Pb
Where P is total pressure.
Pa= partial pressure of A
Ya= mole fraction of A in gas phase
Relative Volatility:
It is defined as the ratio of the partial pressure of 'A' to the mole fraction of A in liquid phase.
Relative volatility is the ratio of A (more volatile component) to the volatility of B. It also known as volatility of A with respect to B and is denoted by a symbol ɑAB