Chemistry Solutions | Basic Chemical concepts
This blog contains Chemistry Solutions | Basic Chemical concepts - Solutions related all Details like Solute solvent Normality Molarity %w/w %w/v ppm Calculations
Blog Content - Chemistry Solutions | Basic Chemical concepts
Solvent Solute Solution
Types of Solutions -
1. Homogenous solution
2. Heterogeneous solution
3. Aqueous solution
4. Ammonical solution
5. Non aqueous solution
6. Gaseous, Liquid, Solid solution
7. Saturated solution
8. Unsaturated solution
9. Supersaturated solution.
Concentration terms -
Normality,
Molarity,
Molality,
Formality,
Mole fraction,
%w/w, % w/v,
PPM
Molarity,
Molality,
Formality,
Mole fraction,
%w/w, % w/v,
PPM
Interview QnA - Chemistry Solutions | Basic Chemical concepts
- What is Solute, Solvent, & Solution?
- What are saturated Unsaturated and Supersaturated solutions?
- What is equivalent weight?
- What is difference between 1 Molarity solution & 1 Molality Solution?
- Why Molarity is reduced with temperature increases?
1. Definitions - Solvent, Solute, Solution
Solvent
The component which present in large quantitySolute
The component present in smaller quantitySolution
It is mixture of solvent + solute = solutionExample -
we are making sugar dissolve in waterSolvent - Water (Larger in quality)
Solute - Sugar ( Less in quantity)
Solution = solvent (water) + solute (sugar)
[ads id="ads2"]
2. Types of solutions
1. Homogenous solution2. Heterogeneous solution
3. Aqueous solution
4. Ammonical solution
5. Non aqueous solution
6. Gaseous, Liquid, Solid solution
7. Saturated solution
8. Unsaturated solution
9. Supersaturated solution.
1. Homogeneous solution
Solution in which two substitutes mixed uniform composition, component can not be identified separately.Example -
Sugar solution in water
2. Heterogeneous solution
Solution in which two or more substances mixed non uniform composition and solution can be identified separately.Example -
Oil mixed in water
We can identify both as separate.
3. Aqueous solution
Solution in which Water is solvent4. Ammonical solution
Solution in which ammonia is solvent5. Non aqueous solution
Solution in which Water is absent6. Gaseous, Liquid, Solid solution
Gaseous solution1. Gas in Gas solution - H2 and O2 solution
2. Liquid in Gas - Water in Air
3. Solid in Gas - Camphor in Air
Liquid solution
1. Gas in liquid - Carbon dioxide in water (soda water)
2. Liquid in liquid - Alcohol in Water
3. Solid in Liquid - Sugar solution
Solid solutions
1. Gas in Solid - H2 palladium
2. Liquid in Solid - Hg in Zn
3. Solid in Solid - Alloys ( Zn in Cu)
7. Saturated solution
- A solution which containing maximum amount of solute
- Solvent can not able dissolve more solute now.
- More addition of solute will be settled down in solution
8. Unsaturated solution
- Solution is able to dissolve more solute.
- Unsaturated solution can still dissolve solute.
9. Supersaturated solution.
- A solution which contains more solute than required at a given temperature.
- Addition of more solute or reduction of temperature will result in form of Crystal.
3. Mole Concept
Definition
1 mole is 6.023 × 10^23 Particles of substance.
Or
Amount of substance containing which are present in 0.012 gram Carbon12
Mole relation to weight of substance and Molecular weight.
Mole = Weight / Molecular weightMolecular weight of Hydrogen is 2 means
Hydrogen
2 Gram = 1 Mole = 6.023 × 10^23 Particles
Calculations
Example - 1
How much moles of H2SO4 in 196 Gram
Molecular weight of H2SO4 = 98
Mole = Weight / Molecular weight.
Mole = 196 / 98 =2
Example - 2
What is weight of 0.2 mole H2SO4?
Mole = 0.2
Molecular weight = 98
Mole = Weight / Molecular weight
Weight = Mole × Molecular Wight
= 0.2 × 98 = 19.6 Gram
4. Normality, Molarity, Molality,Formality, Mole fraction, %w/w, % w/v, PPM
1. Normality
The number of gram equivalent of the solute present in 1 liter of solution at given temperature.
It is indicated by N
Example
1 Normal solution - 40 Gram NaOH (100%) dissolved in 1 liter water will become 1 Normality NaOH solution.
Example
1 Normal solution - 40 Gram NaOH (100%) dissolved in 1 liter water will become 1 Normality NaOH solution.
How it is made?
Gram equivalent weight = 40 Gram
Solute NaOH weight = 40 Gram
Solution quantity = 1 Liter = 1000 ml
As per definition
Normality = (Gram / Liter) / Equivalent weight
Here valent is 1 of OH- in NaOH
So Normality =
(1000 × Solute Weight) / (Equivalent weight × Solution Volume)
= (1000 × 40) / (40 × 1000)
= (1000 × 40) / (40 × 1000)
= 1 Normality NaOH solution.
Normality relation for dilution
N1V1 = N2V2
N = Concentration
V = Volume
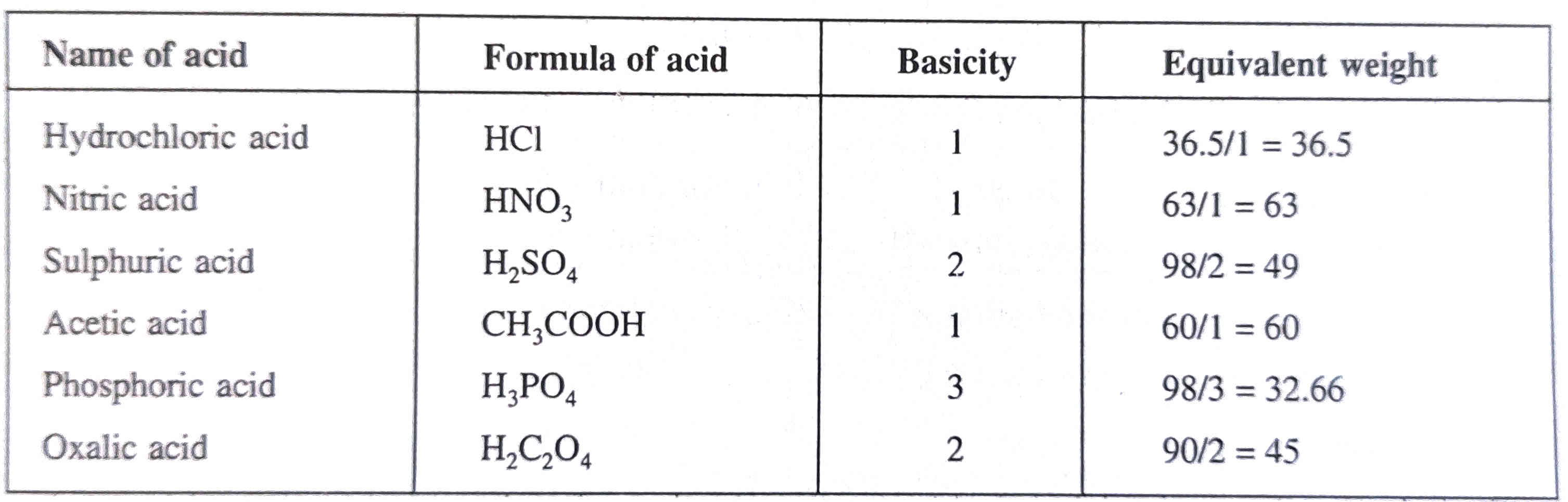
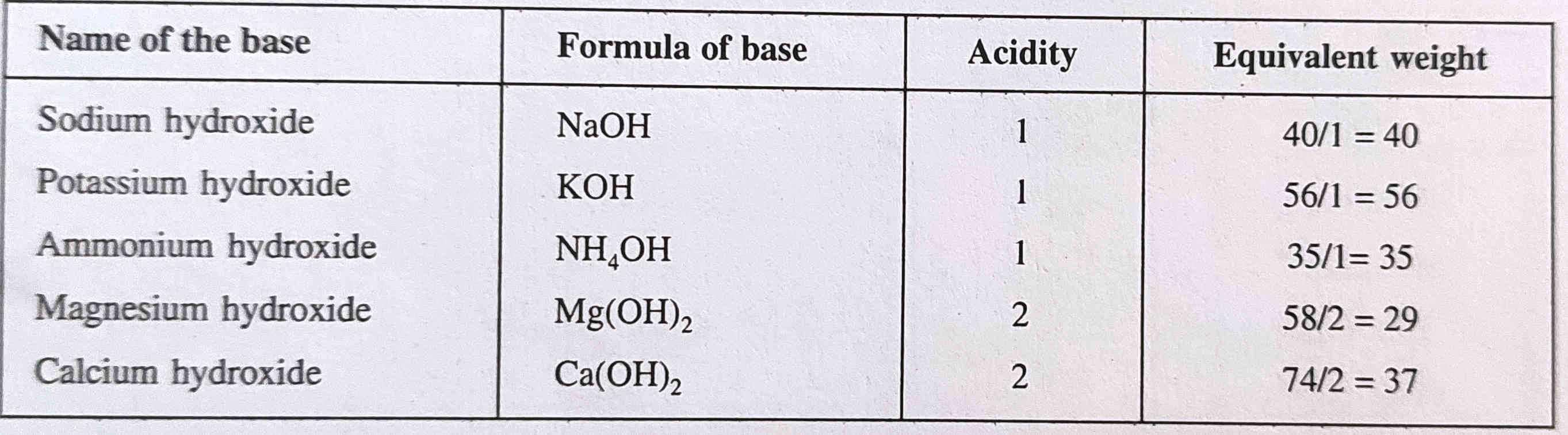
Equivalent weight of substance calculation
Definition - The equivalent weight weight of substance is the weight of it that can react with 1 gram of hydrogen.
It is the ratio of Molecular weight to Basicity of acid, or Acidity of base.
E(Acid or Base)
= Molecular weight / Basicity of acid or Acidity of Base
Here you can see examples of some acids/base with calculations of equivalent weight.
In above equations acidity and basicity can be replaced with
- Total valency of cations, anions or element.
- Numbers of electron gained in Oxidising agent
- Numbers of electron lost in Reducing agent
2. Molarity
When 1 mole of solute dissolved in 1 liter solution at given temperature called 1 Molarity solution or 1 molar solution or 1M solution.
It is indicated by M.
Molarity
= Number of Moles / 1 liter of solutions
= (Weight of substance / Gram molecular weight) / 1 liter solution
= (weight of Substance × 1000) / ( Gram Molecular Weight × Solution quantity)
Relation for dilution
M1V1 = M2V2
M = Molarity of solutions
V = Volume of solutions
Volumatric calculaiton
(M1V1 / n1) = (M2V2 / n2)
n = number of moles in solution
Molarity unit is 'moles/liter' Molarity depends on temperature
Because Charles's law states Temperature is proportional to Volume. So When temperature rises volume also increases and as per molarity equation molarity reduces.
3. Molality
When 1 mole solute weight dissolved in 1 kg solution it is called 1 molality or 1 molal or 1m solution.
Mollity
= (solute mole/ 1 kg solvent) / Molecular weight
= ( 1000 * Solute weight) / (Solute molecular weight * Solution weight)
= (solute mole/ 1 kg solvent) / Molecular weight
= ( 1000 * Solute weight) / (Solute molecular weight * Solution weight)
4. Formality
- Formality of a solution is defined as the number of gram formula masses of the ionic solute dissolved per liter of the solution.
- Solid Ionic solutions are in ionic form so we will call formula weight instead of molecular weight.
- It is indicated by F
- Simply formality showing concentration of ionic solids which does not exists as molecules but exists as network of ions.
- A solution which is containing gram weight according equal to its formula weight in 1000 ml [1 liter] solution is called 1 formality solution or 1 formal solution or 1 F solution.
- Alum solutions like K2SO4.Al2(SO4)3.24H2O Its formula is KAl(SO4)2.12H2O
- If we dissolve 474 Gram alam in 1000 ml [1 liter] water then it will become 1 formality solution of Alum.
- Normality, Molarity, & Formality changes with change in temperature as explained above [Because Charles's law states Temperature is proportional to Volume. So When temperature rises volume also increases and as per molarity equation molarity reduces.
5. Mole fraction
Mole fraction of any substance is defined as the ratio of substance's mole to total moles of solution.
Mole fraction of X-substance = X-substance's mole / Total mole present in solution
Mole fraction of X-substance = X-substance's mole / Total mole present in solution
Total Mole fraction must equal to 1.
Example -
Calculate mole fraction of 80 gram NaOH dissolved in 324 gram water at 25°C
Example -
Calculate mole fraction of 80 gram NaOH dissolved in 324 gram water at 25°C
Mole of Water = Weight / Molecular weight of water
= 324 / 18
Mole of Water = 18 mole
Mole of NaOH = Weight / Molecular weight of water
= 80 / 40
Mole of NaOH = 2 mole
Total mole = Mole of Water + Mole of NaOH
= 80 / 40
Mole of NaOH = 2 mole
Total mole = Mole of Water + Mole of NaOH
= 18 + 2
Total mole = 20 mole
Total mole = 20 mole
Mole fraction of Water = Mole of water / Total mole of solution
= 18/20
= 0.9
Mole fraction of NaOH = Mole of NaOH / Total mole of solution
= 2/20
= 0.1
Total of Mole fractions = Mole fraction of Water + Mole fraction of NaOH.
= 0.9 + 0.1
=1
%W/W - Weight to Weight Percentage
Weight of solute dissolved in 100 gram of solution is called %W/W.
This solutions are made with importance of solute weight.
%W/W = ( 100 * Solute weight ) / ( Weight of Solute + Weight of Solvent )
%W/W = ( 100 * Solute weight ) / ( Weight of Solution )
Example
How much NaOH required to make 500 gram 10 %W/W solution?
%W/W = ( 100 * Solute weight ) / ( Weight of Solution )
Solute weight = ( 10 *500 ) / 100
Solute weight = 50 Gram.
= 18/20
= 0.9
Mole fraction of NaOH = Mole of NaOH / Total mole of solution
= 2/20
= 0.1
Total of Mole fractions = Mole fraction of Water + Mole fraction of NaOH.
= 0.9 + 0.1
=1
6. Weight fraction
Weight fraction is classified in- %W/W - Weight to Weight Percentage
- %W/V - Weight to Volume Percentage.
- PPM - Parts Per Million.
%W/W - Weight to Weight Percentage
Weight of solute dissolved in 100 gram of solution is called %W/W.
This solutions are made with importance of solute weight.
%W/W = ( 100 * Solute weight ) / ( Weight of Solute + Weight of Solvent )
%W/W = ( 100 * Solute weight ) / ( Weight of Solution )
Example
How much NaOH required to make 500 gram 10 %W/W solution?
%W/W = ( 100 * Solute weight ) / ( Weight of Solution )
%W/W = 10 %W/W NaOH10 = ( 100 * Solute weight ) / ( 500 )
Weight of Solute = ? Gram
Weight of Solution = 500 Gram
Solute weight = ( 10 *500 ) / 100
Solute weight = 50 Gram.
So we have to take 50 Gram NaOH(100% Concentration) and then add water till total weight reach 500 Gram.
%W/V - Weight to Weight Percentage
Weight of solute dissolved in 100 millilitre of solution is called %W/V.
This solutions are made with importance of solute weight in Solution volume.
%W/V = ( 100 * Solute weight ) / ( Volume of Solution )
Example
How much NaOH required to make 2000 ml 5 %W/V solution at 25°C?
%W/V = ( 100 * Solute weight ) / ( Volume of Solution )
%W/V = 5 %W/V NaOH5 = ( 100 * Solute weight ) / ( 2000)
Weight of Solute = ? Gram
Weight of Solution = 2000 ml
Solute weight = ( 5 *2000 ) / 100
Solute weight = 100 Gram.
So we have to take 100 Gram NaOH(100% Concentration) and then add water till total volume reach 2000 ml.
PPM - Parts Per Million
It is milligram weight per liter .
Parts Per Million scale is used when solute is too much less quantity.
Oxygen in sea water
Pollution in air.
5. Calculations
Normality calculations
Normality Example-1If We dissolve 2 gram NaOH in 5 liter solution then how much normal solution prepared?
Molecular weight of NaOH = 40 Gram / mole
Solute weight = 2 Gram NaOH
Equivalent weight of NaOH = 40/1 [ Because Acidity of NaOH is 1 due to its valency of OH- is 1]
Solution Volume = 5 liter = 5000 Millilitre
Normality =
(1000 × Solute Weight) / (Equivalent weight × Solution Volume)= 1000 * 2 / 40*5000
Normality = 0.01 N
Normality Example-2
Calculate Normality if 9.8 Gram of H2SO4 dissolved in 2 liter of water.
Molecular weight of NaOH = 98 Gram / mole
Solute weight = 9.8 Gram NaOH
Equivalent weight of H2SO4 = 98/2 = 49[ Because Basicity of NaOH is 2 due to its valency of H+ is 2]
Solution Volume = 2 liter = 2000 Millilitre
Normality =
(1000 × Solute Weight) / (Equivalent weight × Solution Volume)= 1000 * 9.8 / 49*2000
Normality = 0.1 N
Normality Example-3
Calculate the weight of oxalic acid (H2C2O4.2H2O) required to prepare 0.05 normal solution in 2 liter.
Molecular weight of H2C2O4.2H2O = 126 Gram / mole
Solute weight = ? Gram H2C2O4.2H2O
Equivalent weight of H2SO4 = 126/2 = 63[ Because Basicity of NaOH is 2 due to its valency of H+ is 2]
Solution Volume = 2 liter = 2000 Millilitre
Normality = 0.05
Normality =
(1000 × Solute Weight) / (Equivalent weight × Solution Volume)0.05= 1000 * Solute weight / 63*2000
Solute weight = 6.3
Normality Example-4
How much Water will added to 500 ml of 0.05N Na2CO3to get 0.01N solution
Normality equation for dilution
N1V1 = N2V2
N1 = 0.05
V1= 500ml
N2= 0.01 N
V2= ?
V2 = N1V1 / N2
V2 = 0.05 * 500 / 0.01
V2 = 2500 ml
Normality Example-5
How much normality solution of 20ml NaOH required to neutralise 50 ml 0.02N H2SO4?
Normality equation for dilution
N1V1 = N2V2
N1 = 0.02
V1= 50 ml
N2= ? N
V2= 20
N1V1 = N2V2
N2 = N1V1 / V2 = 0.02 * 50 / 20
N2 = 0.05 N
Molarity Calculations
Molarity Example-1How much HCl required to make 2.0 M HCl 500ml solution?
Molarity = = (weight of Substance × 1000) / ( Gram Molecular Weight × Solution quantity)
Molarity = 2 M
weight of Substance = ?
Molecular Weight of HCl = 36.5 Gram/mole
Solution quantity = 500 ml
So
Weight of Substance ( HCl ) = Molarity * Gram Molecular Weight × Solution quantity / 1000
= 2* 36.5*500 / 1000
Weight of Substance ( HCl ) = 36.5 Gram HCl
Molarity Example-2
2 moles of a solute is dissolved in 5 liter of solution What is the Molarity of solution?
Molarity of solution(M) = Number of moles of solute (n) / Volume of solution (V)
Number of moles of solute (n) = 2
Volume of solution (V) = 5 liters of solution.
Molarity of solution(M) = 2 / 5 = 0.4 M
Molarity Example-3
Find the molarity of solution containing 171 gram sugar (sucrose) in 2 liter water?
Molarity = (weight of Substance × 1000) / ( Gram Molecular Weight × Solution quantity)
Molarity = __ M
weight of Substance = 171
Molecular Weight of Sucrose (Molecular formula = C12H22O11) = 342 Gram/mole
Solution quantity = 2000 ml
Molarity = 171*1 / 342*2 = 0.25 M
Molarity Example-4
Find the volume of water required to Prepare 0.1 M H2SO4 from 200 ml 0.5 M solution?
Molarity formula for dilution
M1V1= M2V2
M1= 0.5 M
V1= 200 ml
M2= 0.1 M
V2= ?
V2 = M1V1 / M2
= 0.5*200 / 0.1
V2 = 1000 total volume should be done
Remaining quantity = V2 - V1 = 1000 - 200 = 800 ml water is required.
Molality Calculations
Molality Example-1If we dissolve 0.8 gram NaOH in 2 Kilogram Solvent then what will be molality of solution?
Molality = (weight of Substance × 1000) / ( Gram Molecular Weight × Solution quantity)
Molality = __ m
weight of Substance = 2000
Molecular Weight of Caustic = 40 Gram/mole
Solution quantity = 2000 mg
= 1000 * 0.8/40*2000
Molality = 0.01 m
Mole fraction Calculations
Mole fraction Example-1Calculate mole fractions of solution where 80 gram NaOH dissolved in 324 Gram water?
Mole fractions of Substance = Substance mole / Total mole of solutionsTotal mole = Water mole + NaOH mole
Moles of Water = Weight of water / Molecular weight = 324 / 18 = 18
Moles of NaOH = Weight of NaOH / Molecular weight = 80 / 40 = 2
Total mole = 18 + 2 = 20
Mole Fraction of Water
= Water mole / Total moles of solution
= 18/20
=0.9
Mole Fraction of NaOH
= NaOH mole / Total moles of solution
= 2/20
=0.1
Total of all substance mole fraction must be 1.
Total mole fractions
= Water mole + NaOH mole.
= 0.9 + 0.1
= 1
Weight fraction Calculations
Weight fraction Example-1Calculate NaOH required to make 10% W/W concentrated 500 Gram NaOH?
%W/W = ( 100 * Solute weight ) / ( Weight of Solution )
%W/W = 10 %W/W NaOH10 = ( 100 * Solute weight ) / ( 500 )
Weight of Solute = ? Gram
Weight of Solution = 500 Gram
Solute weight = ( 10 *500 ) / 100
Solute weight = 50 Gram.
Example
How much NaOH required to make 2000 ml 5 %W/V solution at 25°C?
%W/V = ( 100 * Solute weight ) / ( Volume of Solution )
Solute weight = ( 5 *2000 ) / 100
Solute weight = 100 Gram.
How much NaOH required to make 2000 ml 5 %W/V solution at 25°C?
%W/V = ( 100 * Solute weight ) / ( Volume of Solution )
%W/V = 5 %W/V NaOH5 = ( 100 * Solute weight ) / ( 2000)
Weight of Solute = ? Gram
Weight of Solution = 2000 ml
Solute weight = ( 5 *2000 ) / 100
Solute weight = 100 Gram.
So we have to take 100 Gram NaOH(100% Concentration) and then add water till total volume reach 2000 ml.
Read more of Basic Chemical Concept Blogs - Click here
Related Links
Read more on Wikipedia - Click hereNormality, Molarity, Molality Calculator - Calculate now
Buy equipments from thermofisher - Click here
Thanks for reading - Chemistry Solutions | Basic Chemical concepts
Naitik Patel
Industrial Guide
Please Share this to your chemical persons from below