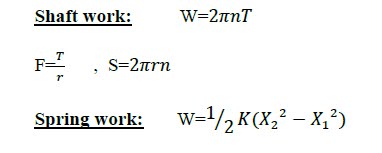Important Definitions
1- Intensive properties:
Are those independent of the size of system such as(Temp., Pressure and density).
2- Extensive properties:
Are those dependent on the size of system such as (Mass, Volume and Energy). Stored Internal Energy
3- Specific properties:
Extensive properties per unit mass Mass & Energy conservation: Einestien Rule: E =MC2 where C is the speed of light =3 ×108 m/s
4- Mass flow rate:
amount of mass flowing across-section per unit time (ṁ).
5- State:
a condition of a system at which all the properties have fixed values.
6- Equilibrium:
implies a state of balance.
7- Thermal Equilibrium:
when temperature is the same throughout the entire system.
8- Mechanical Equilibrium:
there is no change in pressure throughout the entire system.
9- Phase Equilibrium:
the mass of each phase reached an equilibrium value and stayed there.
10- Chemical Equilibrium:
when chemical composition does not change with time.
11- The state postulate:
The state of a simple compressible system is completely specified by two independent intensive properties.
12- Process:
any change that a system undergoes from one equilibrium state to another.
A series of states through which a system passes through a process.
When a process proceed in such a manner that the system remains close to an equilibrium state at all times.
15- Isothermal:
a process during which the temperature is constant.
16- Isobaric:
a process during which the pressure is constant.
17- Isochoric:
a process during which the volume is constant. Thermodynamics can be approached in either of two, usually distinct, ways:
1- Statistical thermodynamics or microscopic or molecular approach
2- Classical thermodynamics or macroscopic approach
Newton’s Laws
1st law:
If the net forces are balanced ( i.e. if the vector sum is zero ), the object will not accelerate
2nd law:
The force is proportional to the rate of change of Momentum
3rd law:
If one object exerts a force on another, then the second object exerts an equal but opposite force on the first.
Energy Classification
1- Stored energy which is contained within the system boundaries. [potential energy ,kinetic energy, internal energy etc. ]
2- Energy in transition which crosses the system boundaries. [Heat, work , electrical energy etc. ]
Transferred Energy
• Heat (Q) Driving potential : temperature difference
• Work - mechanical ( Wm ) Driving potential : unbalance in mechanical forces
• Work - electrical ( We ) Driving potential : voltage difference Intrinsic or internal energy
• Molecular:
- Kinetic ( Uk ) Associated with absolute temperature
- Potential ( Up ) Associated with intermolecular or interatomic forces
• Atomic:
- Chemical ( Uch ) Associated with changes in molecular structure
• Subatomic:
- Nuclear ( UNu ) Associated with changes in atomic structure
The internal energy U = Uk + Up + Uch + UNu
The energy of the system E = PE + KE + U
Total Energy E = 0.5mv2 + mgz+U
Van Der Waals EOS:
Fluids
Compressed Liquid (sub-cooled): Liquid under conditions that’s not about to vaporize.
Saturated Liquid: Liquid which is about to vaporize.
Saturated vapour: Vapour which is about to condense.
Superheated vapour: Vapour which is not about to condense.
Saturation Temperature: the temperature at which a pure substance changes phase at a given pressure.
Latent Heat: the amount of heat released or absorbed during a phase change.
Compressibility factor: a measure of deviation from ideal gas behavior.
Z=1 for ideal gas Z < or > 1 for real gas
Z factor is approximately the same for all gases at the same reduced temperature and pressure(The principle of corresponding states).
Specific Heat: The energy required to raise the temperature of a unit mass of a substance by one degree. (kj ⁄kg.oC)
Heat: is the form of energy that transfers between two systems due to temperature difference (Q KJ).
Adiabatic Process: a process during which there is no heat transfer (Q=0).
Work: is the energy transfer associated with a force acting through a distance (KJ).
Path function: a property which magnitude depends on the path followed during a process as well as end states such as heat & work.
Point function: a property which depends on the state only and not on how the system reached it like volume.
V : Potential Difference
I : Current
Steady flow process: a one the fluid flows through a control volume steadily (No change with time).
Turbine: Work is positive as it’s done by the fluid.
Compressors, Pumps &Fans: Work is negative as it’s from another source and is supplied to the fluid.
Throttling valves: small devices cause a pressure drop which is accompanied with a temperature drop
Joule-Thomson co-efficient: determine the magnitude of that temperature drop.
Isenthalpic action h1=h2.
Kelvin-Planck statement:
‘’It’s impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work’’.
Or, ‘‘No heat engine can have a 100 % thermal efficiency’’
Heat Engine:
A device that converts heat to work.
1. Receives heat from a source.
2. Converts part of it to work.
3. Delivers the waste heat to a sink.
4. Operate on a cycle.
Such as , Gas Turbine and Car Engine.
Steam Power Plant:
Works on a thermodynamic cycle like
(External combustion Engine).
Clausius statement:
‘’It’s impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a lower temperature body to a higher temperature one’’.
Energy efficiency rating EER:
The amount of heat removed from the cooled area in BTU for 1 wh of electricity consumed.
Refrigerators:
Devices that transfer heat from low temperature medium to a high temperature one.
Heat Pump: the same purpose of refrigerators but the reverse objective.